INTRODUCTION
The oral route plays an important role in therapy as it is the most preferred and convenient route for drug delivery systems.1 Gastroretentive drug delivery systems (GRDDSs) are an advanced approach for the novel drug-delivery systems in which the drug is retained in the stomach for a prolonged period.2,3 GRDDSs are particularly suitable for drugs having a narrow absorption window, drugs that act locally in a part of the gastrointestinal tract, drugs that are unstable in intestinal fluids, and drugs that exhibit poor solubility in the intestinal tract.4
Floating drug delivery systems (FDDSs) are one of the most prominent approaches of GRDDs, characterized by the capacity of the formulation to float in and over the gastric contents. FDDSs are low density systems, which allows them to remain buoyant in the stomach for a prolonged period. In the development of FDDSs based on the mechanism of buoyancy the widely employed technology is effervescent systems. In effervescent systems, carbon dioxide gas production occurs due to the reaction of carbonates and bicarbonates present in the formulation with gastric fluid. The gas that forms is entrapped in the polymers, which allows the system to remain buoyant. The FDDSs are effectively used to design sustained drug delivery systems and improve the overall oral bioavailability of drugs.5,6,7
Norfloxacin is fluoroquinolone anti-infective antibacterial drug firstly used in the treatment of urinary tract infections, prostatitis, gonorrhea, and genital tract infections.8 It has 30%-40% bioavailability with a plasma half-life of 3 to 4 h, thus requiring multiple dosing to maintain adequate plasma concentration during treatment.9 Norfloxacin is also poorly absorbed from the lower part of the gastrointestinal tract and it is well absorbed from the stomach. The solubility of norfloxacin in water is pH-dependent, increasing sharply with decreasing pH below 5.10,11
The therapeutic dose of norfloxacin is very high (400 mg orally twice daily) in the treatment of urinary tract infections.12 Many novel approaches have been reported that are used for bioavailability enhancement of norfloxacin, either directed towards the development of a single unit system or unable to produce a significant effect on improvement of bioavailability. Thus it was decided to develop a floating multiparticulate drug delivery system for norfloxacin that could produce sustained release so as to maintain drug plasma levels for improving bioavailability and therapeutic effects.
A floating multiparticulate system was developed in which norfloxacin pellets containing different ratios of sodium bicarbonate (NaHCO3):hydroxypropyl methylcellulose (HPMC) K15M were prepared by extrusion spheronization. The pellets were coated with Eudragit RL 100 on a fluidized bed processor by the bottom spray technique. The amount of the effervescent agent and coating level of Eudragit RL 100 polymeric membrane were evaluated and optimized in terms of floating ability and drug release properties.
MATERIALS AND METHODS
Materials
Norfloxacin was a kind gift provided by Aarti Drugs Ltd, Mumbai, India. Eudragit RL 100 was provided by Evonik, Mumbai, India. All the other chemicals were used as received and were of analytical reagent grade.
Preparation method for norfloxacin pellets
Extrusion and spheronization
The norfloxacin core pellets were prepared using wet granulation by extrusion and spheronization. A powder mixture of norfloxacin and microcrystalline cellulose was mixed in a mortar for 20 min. This was followed by addition of binding liquid consisting of 3% polyvinylpyrrolidone K30 in water. The obtained wet mass was passed through BSS sieve no. 16 to get the extrudates. The prepared extrudates were then transferred to a spheronizer (Shakti Pharmatech, Ahmedabad, India) and spheronized at different spheronizing speeds to get pellets. The prepared core pellets were oven dried overnight at 60°C.
Experimental design
A 3-level, 3-factor, 17-run experimental Box–Behnken design was adopted to optimize levels of variables in the pellet formulations. The selected independent variables were amount of MCC, i.e. microcrystalline cellulose (X1), PVP (K30), i.e. polyvinylpyrollidone (X2), and spheronizing speed (X3) as shown in Table 1. The dependent variables were aspect ratio (Y1) so as to predict the sphericity and hardness (Y2).The generation of experimental runs, ANOVA study and optimization were carried out by Design-expert® software 10. The formulation batches prepared are indicated in (Table 2a).
The optimized norfloxacin pellet batch in terms of sphericity and hardness was selected followed by incorporation of NaHCO3 and HPMC K15M in the ratios of 1:1, 1:2, and 2:1 (w/w) on a dry solid basis as indicated in Table 2b.
Coating of norfloxacin pellets containing NaHCO3:HPMC K15M
The norfloxacin pellets containing NaHCO3:HPMC K15M in the ratio of 1:1 were further coated with Eudragit RL 100 using a fluidized bed processor (ACG, Miniquest-F, Mumbai, India) to obtain weight gain of 5%, 10%, and 15% w/w as shown in Table 2b. The coating solution was prepared by dissolving the desired amount of Eudragit RL 100 in isopropyl alcohol and stirring to obtain a clear solution.
The layering conditions were as follows: batch size, 7.5 g; inlet temperature, 40°C; product temperature, 35°C; air flow, 0.8-1.0 bar; spray pressure, 0.5-0.9 bar; spray rate, 0.130 g/min; and final drying at 40°C for 15 min.
Evaluation of floating norfloxacin pellets
Spectroscopic studies
Calibration curve of norfloxacin in 0.1 N HCl
First 10 mg of norfloxacin was accurately weighed and dissolved in 100 mL of 0.1 N HCl in a volumetric flask to get 100 µg/mL stock solution. This solution was further diluted with 0.1 N HCl to get solutions in the concentration range of 1 to 10 µg/mL. Absorbance of these solutions was determined spectrophotometrically (Shimadzu 1700, Japan) at 273 nm.13,14
Fourier transform infrared spectrum
The powder sample of norfloxacin, Eudragit RL 100, and physical mixture of norfloxacin and polymer (Eudragit RL 100) was kept in a dryer to make it moisture-free. The dry sample of powders was separately mixed and triturated with dry potassium bromide. This mixture was placed in a DRS assembly sample holder. The infrared spectrum was recorded and the spectral analysis was done (Shimadzu, 8400S, Japan).15
Drug content
Norfloxacin pellets equivalent to 400 mg were ground using a mortar and pestle and transferred into a 50 mL volumetric flask containing 0.1 N HCl and the volume was made up to 50 mL. The mixture was sonicated for 10 min to ensure complete extraction of the drug. The solution was filtered through Whatman filter paper and assayed spectrophotometrically (Shimadzu 1700, Japan) at 273 nm to determine the percent drug content.16,17
In vitro drug release studies
Drug release studies of the norfloxacin pellets were performed by USP Dissolution Apparatus-I (Veego DA-8D, India). The dissolution studies were carried out with 900 mL of 0.1 N HCl as dissolution medium at 37±0.5°C and at 50 rpm. Pellets equivalent to 400 mg of norfloxacin were weighed and transferred to the dissolution apparatus. A 10 mL aliquot was withdrawn and immediately replaced by the same volume of fresh medium to maintain sink condition. The aliquot was filtered through Whatman filter paper and absorbance was measured at 273 nm using a UV spectrophotometer (Shimadzu 1700, Japan) to determine the drug release.16,17,18
In vitro buoyancy studies19,20,21
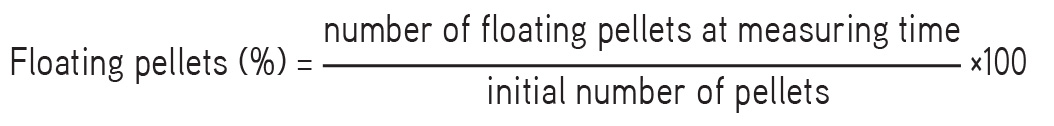
The time required for the pellets to rise to the surface and float as floating lag time and total duration of time for which pellets remain buoyant, i.e. total floating time, were determined. The floating pellets (100) was kept in a USP Type-I dissolution apparatus, the dissolution medium used was 0.1 N HCl, and the conditions were 37±5°C at 50 rpm. The percentage of floating pellets was determined by the following equation:
Scanning electron microscopy
The surface morphology of the optimized coated pellets was examined using a scanning electron microscope. Scanning electron microscopy (SEM) analysis was performed using a Carl Zeiss Supra 5 scanning electron microscope (Germany). The pellet samples were mounted directly onto aluminum stubs and were sputter coated with a gold/palladium mixture for 1 min under an argon atmosphere. The coated pellets were mounted onto the stubs using double-sided adhesive tape.22
Particle size distribution analysis
The size distribution of the gastroretentive pellets was determined using a mechanical sieve shaker (Make-Kumar). A series of BSS standard stainless steel sieves of no. 8, 10, 22, 36, 44, 60, and 100 were arranged in order of decreasing aperture size. An accurately weighed amount of drug-loaded gastroretentive pellets from each batch was placed on the uppermost sieve. The sieves were shaken for 10 min and the material retained on each sieve was weighed separately. A graph of mean size vs % weight retained was plotted to analyze pellet size distribution.23,24
Physical characterization
The micromeritic properties (bulk density, tapped density, Carr’s index, Hausner’s ratio, and angle of repose) of the floating pellets were determined. Friability of the pellets was determined using a USP friability test apparatus. Friability of the pellet formulations was determined as the percentage of weight loss after 200 revolutions of 6.5 g of the core pellets in a friabilator (Roche Friability Tester, India). The hardness of the pellets was determined using a digital hardness tester (Veego, India).25,26,27
Pellet sphericity
Pellet sphericity was determined by measuring the Feret diameter and perpendicular diameter of pellets by vernier caliper. From that aspect ratio was calculated (i.e. ratio of longest Feret diameter and its longest perpendicular diameter).28
RESULTS AND DISCUSSION
UV spectrum of norfloxacin in 0.1 N HCl
The λ max of norfloxacin in 0.1 N HCl was 273 nm. The calibration curve of norfloxacin was obtained in 0.1 N HCl at the respective λ max value as indicated in Figure 1.
Fourier transform infrared spectrum
The IR spectrum of norfloxacin, Eudragit RL 100, and a physical mixture of norfloxacin and polymer (Eudragit RL 100) was obtained by fourier transform infrared (FTIR) (Figure 2). The interpretations of the IR frequencies were done and the absorption bands were consistent with the structure of norfloxacin and Eudragit RL 100. The FTIR spectra of the physical mixture indicated compatibility of norfloxacin and Eudragit RL 100. The FTIR spectra of pure drug showed functional peaks at 3600 to 3250, 1492.95, 2524.46, 1267.27, and 1614.47 cm-1. Eudragit RL 100 IR spectra showed peaks at 2920.32, 1720.56, and 1072.46, while the physical mixture showed peaks at 3491.27, 3365.90, 3012.91, 2850.8, 1745.64, 1610.61, 1456.30, and 1269.20 cm-1 with negligible shift in wave number.
Drug content
The drug content in all pellet formulations was determined by UV spectroscopy and was found to be between 96.75±0.8% and 98.78±0.45%, which indicated that the coating on the pellets also gives good reproducibility of drug content.
Optimization of norfloxacin pellets
To optimize the pelletization process MCC, PVP K30, and spheronizing speed were varied at different levels. Seventeen batches were prepared using a Box–Behnken design, and the aspect ratio and hardness of pellets were determined as response as indicated in Table 3.
The sphericity and hardness of pellets are essential properties to obtain effective coating. Spherical pellets provide a uniform surface, whereas sufficiently hardened pellets can withstand the mechanical stress during the subsequent coating process. The sphericity of pellets was determined in terms of aspect ratio. An aspect ratio value equal to unity indicates spherical pellets. The response surface plots of aspect ratios obtained indicate that increasing the MCC amount and spheronizing speed yields pellets having an aspect ratio near to 1, which is desirable, whereas increasing the amount of PVP K30 yields pellets having an aspect ratio greater than 1. The response surface plots of hardness obtained indicate that with increasing amount of PVP K30 the hardness of pellets also increases, as indicated in Figure 3. From the results of the experimental design batch number B-4 was selected, having aspect ratio 1.1 and hardness 0.59, for incorporation of NaHCO3:HPMC K15M in different ratios and the subsequent coating process.
Regression equations of the fitted quadratic model:
Aspect ratio (Y1) = +1.41+0.036 * A-0.16 * B-0.013 * C-0.16 * A2-0.030 * B2+0.018 * C2-0.030 * A * B-0.038 * A * C+0.13 * B * C.
Hardness (Y2) = +0.48-0.028 * A-0.055 * B+5.000E-003 * C+0.068 * A2+0.16 * B2-0.081 * C2-0.10 * A * B-0.050 * A * C+0.023 * B * C.
Here A, B, and C are spheronizing speed, MCC, and PVP K 30, respectively.
It was observed from the regression equation that the independent variable MCC has a negative effect on the aspect ratio (Y1). This proves that an increasing amount of MCC leads to a decrease in the aspect ratio, i.e. near to unity, which is desirable. On hardness (Y2) a positive effect of PVP K30 was observed. As the concentration of PVP K30 increases the hardness of pellets also increases.
Subsequently, NaHCO3:HPMC K15M was incorporated in the selected batch (B-4) in different ratios, i.e. 1:1, 1:2, and 2:1, to prepare three additional batches (B-18, B-19, and B-20). Drug release and floating studies were conducted on the prepared batches. The batch (B-19) containing NaHCO3 and HPMC K15M in the ratio of 1:2 yielded irregular shape and size pellets due to the higher amount of HPMC K15M, which was difficult to pass through the sieve, and the affecting spheronization process was not studied for drug release and floating behavior.
The plain norfloxacin pellet batch (B-4) showed 87.43% drug release within 1 h. The norfloxacin pellet batch containing NaHCO3 and HPMC K15M in the ratios of 1:1 and 2:1 exhibited 84.19% in 4 h (B-18) and 92.42% in less than 2 h (B-20), respectively, as shown in Figure 4. The drug release in batch B-18 was sustained for 4 h but batch B-20 exhibited higher release in less than 2 h, as it contained more sodium bicarbonate and the generated CO2 gas did not get entrapped in the polymer. The floating lag time for batches B-18 and B-20 was 8 s and 3 s, respectively, in 0.1 N HCl. As the amount of sodium bicarbonate increases the floating lag time decreases. The total floating time of batches B-18 and B-20 was quite short, i.e. 4 h and 2 h, respectively, as shown in Table 4. In batch B-18 the time required to release above 80% of drug and total floating time were 4 h. This type of behavior could be attributed to fact that once the HPMC was dissolved there was no polymeric membrane that could entrap the generated CO2 gas. Hence, batch B-18 containing NaHCO3 and HPMC K15M in the ratio of 1:1 was further selected for coating with Eudragit RL 100 to design complete floating drug delivery system pellets. A Eudragit RL 100 coating was given in order to increase the total floating time and to sustain the release of norfloxacin. Three batches (B-21, B-22, and B-23) were prepared with Eudragit RL 100 coating with weight gain of 5%, 10%, and 15% and evaluated for drug release and floating behavior.
The percentage drug release for batches B-21, B-22, and B-23 was 91.12% in 5 h, and 82.11% and 60.67% in 8 h, respectively, as shown in Figure 5. The drug release studies indicated that as the Eudragit RL 100 polymer coat increases the drug release decreases. The higher coat led to a thicker membrane over pellets, which retarded dissolution medium penetration and hence sustained drug release was obtained. The floating lag time for batches B-21, B-22, and B-23 was 290 s, 440 s, and 795 s, respectively, in 0.1 N HCl. The total floating time of batches B-21, B-22, and B-23 was 5 h, 8 h, and 8 h, respectively, as shown in Table 4. Batches B-22 and B-23 had satisfactory floating ability, with 70%-90% of pellets remaining floating for up to 8 h. The floating studies reveal that an increasing level of polymeric membrane coating increases floating lag time as well as total floating time. Due to the thicker polymer coat water penetration is retarded, which in turn delays CO2 gas generation, leading to increased floating lag time. However, once the CO2 gas is generated the increasing amount of polymer coat inhibits the permeation of gas out of the floating pellets system and maintains the buoyancy for a longer period.
Among the three complete floating drug delivery system pellet batches B-21, B-22, and B-23, batch B-22 was found to be optimized based on the criteria of attaining minimum floating lag time (less than 10 min), maximum total floating time, and maximum value of drug released in 8 h.
Scanning electron microscopy
The surface morphology of the norfloxacin uncoated pellet batch (B-4) and coated pellet batch (B-22) was studied through SEM. The uncoated norfloxacin pellets’ surface was wrinkled and rough, whereas the polymer-coated pellets showed smoother surfaces as indicated in Figures 6a and 6b.
Particle size distribution analysis of pellets
The particle size distribution analysis of pellets indicates a narrow size distribution in which most of the pellets are in the size range of 1000 µm to 1200 µm, as shown in Figure 7.
Physical characterization of pellets
From the physical characterization of pellets, it was clearly observed that all the batches have excellent flow properties, with an angle of repose in the range 25.5±0.49° to 28.02±0.30° and Carr’s index and Hausner’s ratio in the range of 5% to 15% and 1.05±0.3 to 1.14±0.3, respectively (Table 5). The aspect ratio of pellets obtained was near to unity. Hardness and friability were in the range of 0.49±0.01 to 0.61±0.01 kg/cm2 and 0.17±0.52%, respectively.
CONCLUSIONS
A gastroretentive multiparticulate drug delivery system for norfloxacin based on the gas generation technique was successfully designed and developed. The identification and purity of drug were affirmed by conducting infrared and UV spectroscopy studies. A 3-level, 3-factor, 17-run experimental Box–Behnken design was employed to optimize the norfloxacin pellets in terms of sphericity and hardness required to attain effective coating subsequently. The pellet batch obtained at spheronizing speed 950 rpm containing 35% MCC with 6% PVP K 30 produced pellets with the desired sphericity and hardness. NaHCO3 and HPMC K15M in the ratios of 1:1, 1:2, and 2:1 (w/w) on a dry solid basis were incorporated into the norfloxacin pellets and they were further coated with Eudragit RL 100 using a fluidized bed processor to obtain weight gain of 5%, 10%, and 15% w/w. The floating ability and in vitro drug release of the system were dependent on the ratio of NaHCO3 to HPMC K15M and the percentage of Eudragit RL 100 polymer coat. As the amount of sodium bicarbonate increases floating lag time decreases. The drug release studies indicated that as the Eudragit RL 100 polymer coat increases the drug release decreases, producing sustained release of norfloxacin. The floating multiparticulate pellet batch containing NaHCO3 and HPMC K15M in the ratio of 1:1 with 10% Eudragit RL 100 coating showed the minimum floating lag time (<10 min) and 82.11% average drug release in 8 h. The floating study reveals that 70%-90% of pellets remained floating for up to 8 h. The significant result obtained with the study was that a floating multiparticulate drug delivery system based on the effervescent mechanism can be effectively employed for improvement of the bioavailability and therapeutic effect of drugs having poor absorption in the lower part of the gastrointestinal tract.
ACKNOWLEDGEMENTS
The author acknowledges Aarti Drugs Ltd, Mumbai, India for providing the gift sample of norfloxacin. The author is also thankful to Savitribai Phule Pune University for conducting the scanning electron microscopic studies.
Conflict of Interest: No conflict of interest was declared by the authors.