INTRODUCTION
In recent years, solid lipid nanoparticles (SLNs) have received a lot of attention in drug delivery systems with the aim to improve solubility and bioavailability and to provide controlled delivery of drugs.1 They are submicron colloidal carriers. The particle size varies in a wide range between 50 and 1000 nm.2 They are biodegradable and biocompatible, possess low toxicity, and are preferred as potential carriers for a wide variety of poorly soluble drugs. The solid lipid core matrix of SLNs can be used to solubilize lipophilic molecules. They are characterized by their unique properties of smaller size, large surface area, and high drug loading. They have higher potentials than polymeric nanoparticles, fat emulsions, micelles, and liposomes. The suitability of SLNs as carriers is seen in the improvement of biopharmaceutical parameters of poorly soluble drugs, mainly enhancement of bioavailability, drug stability, drug targeting and thereby minimizing toxicity. From the manufacturing point of view they offer limited or no use of organic solvents and ease of scale up for large-scale production.3,4,5
Azithromycin dihydrate (AZT) is a new generation macrolide antibiotic. It is an azalide with a 15-membered azalactone ring. It is derived from erythromycin and possesses enhanced antimicrobial activity. It is prescribed for once daily dosing because of its long half-life. According to the Biopharmaceutical Classification System, AZT can be classified as a class II drug; therefore, the rate-limiting step in the process of drug absorption is the dissolution of the drug, which accounts for its low bioavailability.6,7 Furthermore, it is a substrate of the p-glycoprotein transport system, which is further responsible for its low bioavailability due to ileal clearance (biliary plus intestinal excretion clearance).8,9
Therefore, the present investigation aimed to prepare and characterize azithromycin loaded SLNs using stearic acid with different surfactant combinations with a view to improve the solubility of AZT. It can be used as an alternative carrier transport system to improve the dissolution and bioavailability of AZT.
MATERIALS AND METHODS
Materials
AZT was a gift sample from Strides Ltd, Bangalore. Stearic acid was procured from Loba Chemicals Pvt. Ltd, Mumbai, India. Tween 20 was procured from SD Fine Chem Ltd. Poloxamer 188 and poloxamer 407 were obtained from Dr. Reddy’s Laboratories. All other reagents and solvent used were of analytical grade.
Methods
Preparation of AZT SLNPs by high shear homogenization
The SLNs of AZT were prepared using stearic acid and different surfactants in varied proportions by high shear homogenization.10 Table 1 reports the composition of AZT loaded SLNs. The selected lipid and the different surfactants were varied in ratio from 1:1 to 2:1. Melting of stearic acid was carried out above its melting point. The drug was dispersed in the molten lipid. A solution of surfactant in distilled water at the same temperature of the molten drug lipid mixture was added to the drug lipid mixture and emulsified by a high shear homogenizer (Polytron PT 1600E Kinematica AG, Switzerland) at 25,000 rpm for 20 min. The nanoemulsion thus formed was subjected to cooling at room temperature. Azithromycin loaded SLNs were finally obtained and stored in a desiccator for further evaluation.
Evaluation of solid lipid nanoparticles
Drug content
For determining drug content, 250 mg of SLN was weighed accurately and dissolved in phosphate buffer pH 6.0 up to 250 mL. Then 1 mL was taken and diluted to 100 mL with phosphate buffer pH 6.0 and the solution was analyzed spectrophotometrically at 482 nm using 13.5 mol/L sulfuric acid as color developing agent. Percentage drug content was calculated using the formula;11

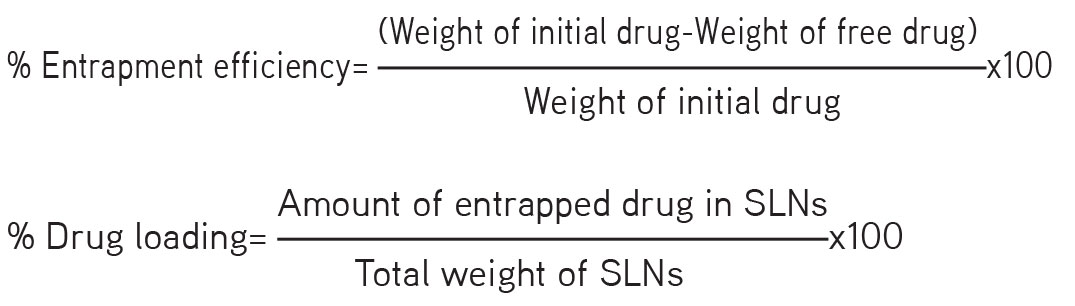
Determination of entrapment efficiency and drug loading
The entrapment efficiency was determined by centrifugation. SLN dispersion equivalent to 10 mg drug was centrifuged at 15,000 rpm for 60 min using a Remi cooling centrifuge (Mumbai, India). The supernatant layer was diluted with phosphate buffer pH 6.0 and the absorbance was measured at 482 nm in a ultraviolet visible spectrophotometer (Shimadzu 1800, Japan) using 13.5 mol/L sulfuric acid as color developing agent.12 The percentage entrapment efficiency and percentage drug loading were calculated from the following equations:13,14
In vitro dissolution studies
The in vitro release of different SLN dispersions of azithromycin was carried out using a USP type II apparatus (paddle type). Formulation equivalent to 250 mg of drug was taken in the dissolution chamber containing 900 mL of phosphate buffer pH 6.0. The temperature of the dissolution chamber was maintained at 37±0.5°C, samples were withdrawn at predetermined intervals for 45 min, and the same were replenished with fresh buffer to maintain the sink condition. The drug content of each sample was determined spectrophotometrically at 482 nm after suitable dilution with phosphate buffer pH 6.0 using 13.5 mol/L sulfuric acid as color developing agent. The amount of drug released from the nanoparticles was calculated.15
Particle size analysis
The mean particle size and polydispersity index (PDI) were determined by dynamic light scattering (Zetasizer nano ZS, Malvern Instruments, UK). Samples after appropriate dilutions in Milli Q water were taken for analysis.16 Particle size analysis for the formulations was carried out following proper dilutions in Milli Q water at 25.1°C with equilibration time 70 s in triplicate.
Zeta potential analysis
Electrophoretic light scattering was used to achieve the electrophoretic mobility of nanoparticles using a Zetasizer nano ZS (Malvern Instruments, UK). Measurements were carried out in triplicate at 25.1°C using water as a dispersant (refractive index: 1.330) in a clear disposable zeta cell.
Scanning electron microscopy
To study the surface morphology scanning electron microscopy was used.17 The study was carried out at low accelerating voltage of about 15 kV with load current about 80 mA and working distance WD=9.1 mm using a standard error mean (SEM) (Model JSM 840 A, Jeol, Japan).
Fourier transform infrared spectroscopy
The compatibility study of AZT, stearic acid, and the other surfactants was performed by attenuated total reflection (ATR) at ambient temperature using a Bruker Model Alpha E (USA) through direct sampling. The microfine powered drug was sprinkled on the ATR crystal. This facilitated the refraction. The fourier transform infrared spectroscopy (FTIR) spectra of the physical mixture of drug and excipients thus obtained ascertained the compatibility of the drug with the excipients.18
Stability studies
The nanoparticles of AZT were stored in capped glass vials at 40±2°C/75% RH±5% RH for 90 days. Samples were evaluated periodically for particle size, drug content, and % release at the end of 30, 60, and 90 days.14
Differential scanning calorimetric study
The differential scanning calorimetric (DSC) thermograms of pure drug, stearic acid, blank formulation, and AZT loaded SLNs were recorded using a Mettler-Toledo differential scanning calorimeter (Mumbai, India). A suitable quantity of sample was weighed and heated in a closed pierced aluminum pan at a scanning rate of 10°C/min between 30 and 200°C and with 20 mL/min nitrogen flow.
RESULTS AND DISCUSSION
Drug content
The drug content of nanoparticles of AZT was in the range of 88.03% to 97.86%. The high shear homogenization method for preparing SLNs of azithromycin with varied proportion of stearic acid and different surfactants at different concentrations was found to be effective. The results are summarized in Table 2.
Entrapment efficiency and drug loading
To achieve high entrapment of drug in the different concentration of lipid matrix of stearic acid, the type and concentration of surfactants were varied in the ratio from 1:1 to 2:1. It was observed that as the drug was moderately lipophilic the entrapment efficiency of the drug in the matrix was highly satisfactory. The range of the percentage entrapment and drug loading was in the range from 69% to 89% and 23% to 30%, respectively, and is summarized in Table 2.
Cumulative drug release
In vitro release of the AZT loaded nanoparticles in phosphate buffer pH 6 was studied in a USP type II dissolution apparatus over 45 min. Data from the percentage release of drug from the SLNs are illustrated in Figures 1, 2, and 3. Formulations (F1, F5, F9, F4, F8, and F12) containing high lipid surfactant ratios showed less drug release within 45 min. This may be attributed to the hydrophobicity of the matrix and affinity of the drug to the lipid component. Formulations containing relatively high amounts of surfactant (F3, F7, and F11) showed improved dissolution probably due to a reduction in interfacial tension with the increase in surfactant concentration. Better release was obtained from formulations with 1:1 ratio of lipid and surfactant. Formulations F2, F6, and F10 showed better release than any other formulations. This may be attributed to the effect of the concentration of surfactant on the physical properties of the nanoparticles. Poloxamer 188 was the best probably due to its low molecular weight and thereby low viscosity and high HLB compared to other surfactants, which enhanced the release of drug from the lipid matrix.19,20
Particle size, PDI, and zeta potential measurement
AZT loaded SLNs that showed the highest entrapment efficiency and drug release were subjected to further characterization of particle size, zeta potential, and PDI as shown in Table 3. The selected formulations showed a mean particle size between 143 and 167 nm. The particles were in an acceptable nanometer range, favored for lymphatic uptake. A PDI less than 1 indicated that the formulations were monodisperse in the system. Estimation of zeta potential helps in determining surface charge and potential stability of the dispersed system. Usually high positive or negative zeta potential is required for SLNs as the same charge results in electrostatic repulsion and thereby avoids aggregation of particles. The zeta potential of the selected formulations F2, F6, and F10 was -30.1 mV, -31.8 mV, and -30.6 mV, respectively.
Scanning electron microscopy
The surface morphology of the SLNs was studied by SEM. Formulations F2, F6, and F10 were subjected to size and morphology studies. The photographs (Figures 4, 5, and 6) revealed that all particles were discrete entities, slightly spherical with a smooth surface. Thus the employed method of preparation of SLNs by hot homogenization was found to be appropriate for formulation of nanoparticles.
FTIR studies
The FTIR spectrum of pure AZT showed the characteristic peaks at 2971.30 cm-1 (C-H stretching), 1718.56 cm-1 (C=O ketone), 1375.53 cm-1 (C-H deformation in alkane), and 1186.97 cm-1 (C-O-C ether stretching).
The FTIR spectra of the 1:1 physical mixtures of drug, stearic acid, and surfactants had all the characteristic peaks (Figure 7) and the band values of AZT confirming that all the functional groups were well preserved.
This study clearly indicated the absence of any chemical interaction between the drug and the excipients, and they were compatible with each other.
Stability studies
The selected formulations were subjected to short-term stability studies for 90 days at 40±2°C/75%±5% RH. Both physical and chemical changes were observed during the study at an interval of 30 days. Physical stability was analyzed in terms of particle size, whereas chemical stability was analyzed by the determination of drug content and change in the release profile. The drug content was found to be 93.21%, 97.53%, and 97.11% for formulations F2, F6, and F10, respectively, at the end of the study. Figures 8 and 9 reveal that all the formulations retained their size and release profile during the study period. Therefore, the formulations were found to be stable at 40±2°C/75%±5% RH.
DSC studies
DSC was used to investigate the thermal behavior of the pure drug in the lipid surfactant mixture. AZT showed an endothermic peak at 115.77°C with onset at 93.11°C and end set at 125.54°C corresponding to the melting point of AZT as shown in Figure 10a. It was noted that there was a shift in the melting point from 115.77°C to 47.07°C (Figure 10b), indicating that AZT must be molecularly dispersed in the formulations.21 The complete fusion of drug in the matrix was further proved by the thermograms of pure stearic acid and the blank SLN corresponds to F6 as shown in Figure 11.
CONCLUSIONS
This study demonstrated that AZT loaded SLNs were successfully prepared and characterized. All the formulations showed improved dissolution of AZT with satisfactory entrapment efficiency and drug loading. The characterization of formulations in terms of their particle size, zeta potential, PDI, surface morphology, and enhancement of dissolution proved the suitability of high shear homogenization to entrap AZT successfully in SLN carriers. From this study it can be concluded that poloxamer 188 is the best surfactant among the three surfactants used for improvement of solubility of AZT in the formulation of SLNs.
ACKNOWLEDGEMENTS
The authors are grateful to the management and principal of Krupanidhi College of Pharmacy, Bangalore, for providing the support and facilities to carry out the investigation. We extend our gratitude to IISC Bangalore and Aimil Limited, Bangalore, for their support. We are grateful to Dr. Reddy’s Laboratories, Hyderabad, and Strides Arcolab, Bangalore, for their generous contribution of chemicals and drugs.
Conflict of Interest: No conflict of interest was declared by the authors.