INTRODUCTION
One of the common immune-based chronic autosomal diseases is psoriasis, which may develop in people of any age. It is also known as a genetically influenced inflammatory disease in skin that is identified as salmon-colored plaques covered by loosely adherent scales that are silver white. This disease spreads to the whole body in a couple of days and causes total body erythema with scaling known as erythroderma. The disease most frequently affects the joints like the skin of the elbow, knees, scalp, lumbosacral areas, intergluteal cleft, and glans penis.1 People are neglecting this dermatitis but it may sometimes be associated with arthritis, myopathy, enteropathy, spondylitic heart disease, diffuse cutaneous and mucosal pustules, and electrolyte disturbances.2 Hence, appropriate treatment is required to cure psoriasis at root level. A vast number of allopathic drugs are available for the treatment of psoriasis. Some drugs, i.e. lithium, β-blockers, and chloroquine, are provocative factors3 and many drugs are associated with various side effects. Therefore, currently the importance of using natural herbs is emphasized for the treatment of skin diseases like psoriasis either in combination or alone in different forms. Whole parts of natural plants such as the root, bark, stem, seed, flowers, or leaves are effective for their versatile therapeutic activities. In the present study Calendula officinalis and Phlebodium decumanum leaves were selected based on the traditional knowledge. C. officinalis (CO) (Family: Asteraceae), commonly known as the pot marigold, is abundantly available throughout India and is cultivated in most soils in a sunny climate. The leaves contain carotenoids such as lutein (80%), zeaxanthin (5%), and beta carotene.4,5 Apart from that, the leaves also include polyphenols, alkaloids, steroids, tannins, and flavonoids.6 Many applications are reported with the flowers, whereas traditionally the leaves are used for wound healing and treatment of burns and infections, mainly due to the presence of essential phytoconstituents in the leaves. Scientifically the leaves are also stated to have antimicrobial,7 hepatoprotective,8 and wound healing activity.9 P. decumanum (PD) (family Polypodiaceae), commonly known as the ornamental fern, is abundantly available in damp regions in many parts of India.10 The leaves contains many chemicals, i.e. alkaloids; various fatty acids like oleic acid, linoleic acids, linolenic acids, arachidonic acid, eicosapentaenoic acid, and elaidic acid; arabinopyranosides; ecdysone; ecdysterone; juglanin; kaempferols; and melilotoside.11 Our literature survey revealed the presence of anti-inflammatory,12 antioxidant,13 wound healing, immune system improving,14 antimicrobial, anthelmintic,10 etc. activity. The therapeutic activities of plant constituents greatly depend on soil fertility, climatic conditions, and content of metal ions in the accumulated plant parts.15,16,17,18,19 Many studies have reported various activities based on the effectiveness of either extracts in combinations or with isolated compounds but there are very few reports on the impact of soil fertility and content of soil metal ions and their uptake by the plant foliage on therapeutic efficacy. No such literature is available on the relation with metal ion content and activity of the plants selected in this investigation. Therefore, in the present study C. officinalis and P. decumanum leaves were selected from the West Bengal and Karnataka zones of India for establishment of effective treatment as well as the impact of foliage metal ions against psoriasis.
MATERIALS AND METHODS
Selection of experimental zones
In the present investigation, the hilly region of Darjeeling, West Bengal, and the Nandi Hills, Bangalore, Karnataka zones were selected for collection of leaf samples of the said plants because of the soil nature and the natural habitat of the plant species. The hilly region’s soil is highly acidic, whereas the soil of the Nandi Hills is slightly basic but both are hill areas (Figure 1). The Terai region lies between latitude 26°30′30″ to 27°8′45″ N 88° and 88°56′15″ E longitude, whereas Bangalore lies between latitude 12°58′38″ N and longitude 77°35′14″ E in which the Nandi Hills are located at latitude 13.3667° N and longitude 77.6833° E. The average annual rainfall of Darjeeling and Bangalore is about 2547 mm and 870 mm, respectively.
Authentication and preparation of plant samples
The leaves of the said plants were taxonomically identified and authenticated by Dr. Rajasekharan PE, Principal Scientist, Department of Plant Biotechnology, Indian Institute of Horticultural Research, Bangalore. The voucher specimens of both leaves collected from West Bengal and Karnataka (KCP/34/WB-PD/2016-17; KCP/35/WB-CO/2016-17; KCP/36/KAR-PD/2016-17 and KCP/37/KAR-CO/2016-17) have been deposited in the herbarium section of the Pharmacognosy Department of Krupanidhi College of Pharmacy, Bangalore, for future reference.
The leaves were collected in June 2016 from both places and transported in sealed plastic containers to the laboratory for processing. The leaves were cleaned with running tap water and oven dried at 60°C for 2-3 h. Shade drying was not recommended because during the rainy season the moisture content in the environment was high and there was more possibility of microbial growth rather than drying. After oven drying the leaves were blended in a mixer grinder into a coarse powder and separately kept in air tight sealed plastic containers, labeled properly for further investigation.
Analysis of soil samples for metal ion content
Total metals and diethylenetriaminepentaacetic acid (DTPA) extractable metals (iron: Fe; copper: Cu; zinc: Zn; lead: Pb; cadmium: Cd; nickel: Ni; arsenic: As; and chromium: Cr) were determined with the help of an atomic absorption spectrophotometer (AAS, PerkinElmer model: AAnalyst 100; Australia) by acid digestion method. Next 10 g of soil sample was taken in a conical flask and 20 mL of 0.005 M DTPA (0.005 M DTPA; 0.1 M triethanol amine and 0.01 M CaCl2, 2 H2O) was added to it. Then it was shaken for 2 h on a mechanical shaker and it was filtered with Whatman No. 42 filter paper. Then the filtrate was determined for various metal contents in different soils. Blank samples were also prepared for correction. All the samples were checked by carrying out triplicate analyses for the reproducibility of the method used.
Analysis of leaf samples
Leaf samples were pretreated with concentrated nitric acid in a digestion flask followed by mixing with acid mixtures. Digestion was carried out at 200°C until dense white fumes of H2SO4:HClO4 were evolved and finally white residue was obtained. Subsequently the digested samples were diluted with deionized water and the volume made up to 50 mL. Final solutions were analyzed for various heavy metal contents (Cd, Cr, Cu, Fe, Ni, Pb, and Zn) using an AAS (PerkinElmer model: A Analyst 100; Australia). Air acetylene was used as the common oxidant/fuel combination gas in the AAS and the concentration of the above elements was determined using the standard condition. The wavelengths were selected for the analysis based on the concentration ranges of the sample and the linear relation between the absorbance (AU) and concentration of the determined element. Blank samples were also prepared for correction. All the samples were checked by carrying out triplicate analyses for the reproducibility of the method used.
Preparation of plant extracts and their phytochemical screening
Stored coarsely powdered samples (250 g) were used for the preparation of extracts by direct reflux method using distilled water as solvent at 45°C for 8 h. Thereafter extracted liquids were filtered with Whatman No. 1 filter paper and evaporated with a rotary flash evaporator at 45°C and stored in refrigeration condition (at 4°C) in glass bottles for further experimentation. The yield of extracts was calculated and then the presence of various phytochemicals was screened qualitatively by various chemical tests for the detection of constituents like alkaloids, flavonoids, steroids, tannins, glycosides, terpenoids, and others by following standard methods.20,21
Selection of animals
Healthy albino mice (50-70 g) obtained from Krupanidhi Pharmacy institutional animal housing facilities were used for the present investigation. The animals were housed in polypropylene cages and were left for 7 days for acclimatization to the animal room and they were kept under controlled conditions (12 h light/dark cycle at 22±2°C) and fed on standard pellet diet and water ad libitum. All animals were taken care of ethically as per the guidelines of CPCSEA with approval from the Institutional Animal Ethics Committee (KCP/PCOL/06/2017).
Acute dermal toxicity
Acute dermal toxicity studies were carried out using albino mice in accordance with the Organization for Economic Cooperation and Development guidelines no. 402.22 The mice (six animals per group) were divided into two groups. The animals’ hair was removed from the dorsal portion of the body surface and a dose of 2000 mg/kg body weight for two different extracts was applied. The animals were observed and recorded for changes of redness, erythema, sleep pattern, behavior pattern, and mortality for 14 days. Thereafter a skin irritation test was also carried out with the aqueous extracts over 72 h.
Grouping of animals and experimental method
Based on the toxicity study, the following groupings of animals were carried out (Table 1). Group I is normal (untreated), while group II received standard drug, Retino-A 0.05% (Tretinoin cream U.S.P.) - Janssen-Cilag Pharmaceuticals (Trademark of Johnson & Johnson, USA) in cream form (positive control). The group III to VIII mice were administered a daily topical dose of 62.5 mg of 5% imiquimod cream (IMQ, Aldara; 3M Pharmaceuticals, UK) to a 3 cm×4 cm shaved area on their backs for 7 consecutive days and they were observed for induced psoriasis.
An objective scoring system was applied based on the clinical psoriasis area and severity index.23 Redness, erythema, and scales were scored independently on a scale from 0 to 4: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked. The cumulative score (sum of redness, erythema, and scaling) served as a measure of the psoriasis severity index (PSI) (scale 0-12).24 After induced psoriasis from day 8 onwards extract treatment was started once daily, 5 times a week, for 21 days. At the end of the study, the animals were anesthetized using high dose carbon dioxide gas in a closed desiccator. Skin specimens were collected and preserved in glass vials containing 10% formalin solution for histological examination. Longitudinal sections of each mice skin specimen (about 5 mm diameter and 5 µm thickness) were prepared by microtomy and stained with hematoxylin and eosin (H and E) dye for histological examination.
Statistical analysis
The experimental results were represented as mean ± standard deviation and analyzed using one-way analysis of variance by Tukey–Kramer multiple comparisons test. The statistical calculations were performed using GraphPad 5 software (San Diego, CA, USA). P<0.05 was considered statistically significant in all the groups. Risk assessment code of metals in the soil was performed following the procedure described by Singh et al.25 as:
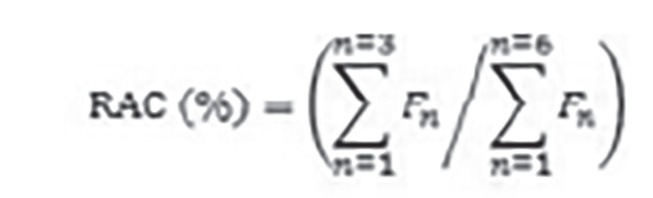
Here “Fn” is the concentration of metal in the “nth” fraction.
RESULTS
Analysis of soil samples for metal ion content
Soli samples were collected from both geographical locations and analyzed for preliminary soil tests like soil pH, organic carbon, and color of soil. Then total metals and DTPA extractable metals were analyzed by AAS. The results revealed very high acidic soil (pH 4.32) in the Darjeeling region compared to the Nandi Hills soil (pH 5.20). All other results are tabulated in Table 2.
Analysis of leaf samples for metal ion content
Collected leaf samples were also analyzed for uptake of metals separately by AAS and the results revealed that leaf samples collected from the Nandi Hills, Bangalore, contained more metal ion uptake by the leaves. The results are tabulated in Table 3.
Yield of the extracts and phytochemical screening
Yield of the aqueous extracts from both collection areas was calculated and the results are shown in Figure 2. The results revealed that the yield of leaf samples of PD and CO collected from the Bangalore zone was higher than that of samples collected from the Darjeeling, West Bengal zone.
Percentage yield was calculated and it was found that the PD sample was higher (11.48%) than the CO sample (9.68%) collected from the Nandi Hills region and the same trend was followed for samples collected from Darjeeling. The PD sample showed a percentage yield of the leaf sample of 10.52%, whereas for CO extract it was 8.8%.
Phytochemical screening with respect to chemical tests was carried out and revealed the presence of various group of phytochemicals in all four leaves’ aqueous extracts, which is depicted in Table 4.
Acute dermal toxicity
The study revealed the aqueous leaf extract of both CO and PD are nontoxic when tested at maximum dose levels of 2000 mg/kg body weight. Neither mortality nor any sign of toxic reactions was found during the study period. Furthermore, no skin irritation was observed with the applied extracts even after 72 h of study.
Antipsoriatic activity
Topical application of 62.5 mg of 5% imiquimod was performed for 7 days and resulted in the development of induced psoriasis in each group of mice (groups II-VIII). After 3-4 days, the back skin of the mice started to display signs of erythema, scaling, and thickening (Figure 3).
Various changes such as redness, erythema, and silvery scales on the exposed area were marked visually and found an increase up to day 7 and the cumulative score, PSI, was significantly (p<0.05) increased as indicated in Table 5 and Figure 4.
After day 7, from day 8 onwards up to 3 weeks the extracts were applied topically to groups II-VIII. The severity of psoriatic lesions was evaluated by visual and histological studies. In group II, topical application of Retino-A cream (0.05%) reduced (p<0.05) the severity of redness, erythema, and scales from days 7 to 21. Thereafter a drastic reduction (p<0.01) in phenotypic changes like redness, erythema, and scales was observed for groups VII and VIII, in which combined extracts were applied and the results showed a dose-dependent manner. Among the responses, the plants procured from the Nandi Hills, Bangalore, Karnataka state, showed more significant results (Tables 6, 7, 8, 9) than samples procured from Darjeeling, West Bengal state (Tables 10, 11, 12, 13). Combined extracts at 200 mg/kg b.w. resulted in a more significant PSI score (p<0.01) on day 14 as well as on day 21 than the later geographical zone. Interestingly the results were better in terms of reduction of redness, erythema, scales, and cumulative score in animals, which showed the therapeutic efficacy of the selected plant samples on induced psoriasis compared to the standard drug applied.
Histopathological study
The histological examination showed an increased epidermal thickness, hyperproliferation of keratinocytes granulocyte infiltration, the presence of microabscesses, and capillary loop dilatation in IMQ-induced mouse skin as compared to normal mouse skin (Figures 5a, 5b). Thereafter the epidermal thickness of extract-treated animals was compared with that of untreated animals, which showed a remarkable decrease in thickness compared to the applied standard (Figure 6). Thickness of the epidermis cell was significantly less (26.18 µM) (**p<0.01) when combined PD and CO extracts were applied at 200 mg/kg b.w. compared to the standard (40.14 µM) in terms of reduced epidermal thickness, hyperproliferation, granulocyte infiltration, the presence of microabscesses, and capillary loop dilatation (Figures 7a-c).
Correlation coefficient
The data were analyzed for correlation between uptakes of essential metals in leaves and reduction in epidermal thickness in psoriasis treatment. The results revealed high significance. PD and CO leaf samples procured from the Nandi Hills showed better uptake of Fe (2.05 mg/kg and 1.88 mg/kg, respectively), Cu (0.78 mg/kg and 0.62 mg/kg, respectively), and Zn (1.12 mg/kg and 0.98 mg/kg, respectively) than Darjeeling samples. Extracted plant samples were calculated for percentage of extracts and the results revealed that the Nandi Hills samples gave more extract due to their higher content of metallic ions (Figure 2). These metal contents further correlated with the reduction in epidermal thickness, which showed significant results. The increased content of metals in PD and CO leaf samples decreases the epidermal thickness (94.33 µM and 97.30 µM, respectively, on day 21) (Table 14) more than samples procured from Darjeeling, West Bengal (98.10 µM and 100.20 µM, respectively, on day 21).
DISCUSSION
Metal ion content in soil and leaf sample
The results (Table 2) show that the pH and organic carbon content in the Darjeeling soil were 4.32 and 0.64%, while in the Nandi Hills soil they were 5.18 and 0.32%, suggesting that the soil of Darjeeling was more acidic compared to the Nandi Hills soil. As regards the organic carbon content, it was observed that the amount was much higher (0.64%) in the Darjeeling soil compared to the Nandi Hills soil (0.32%), which might be due to variation in climatic conditions, especially in temperature. The prevailing temperature in the Darjeeling region was much lower compared to the Nandi Hills, which might be explained by the lower loss of organic carbon in the former region resulting from the very little oxidation of organic carbon from the soil compared to that of the latter Nandi Hills soil, causing a greater rate of oxidation of organic carbon.
As regards the total and available heavy metal content in soils, it was found that the amount of both total and available metal concentration was always higher in the Nandi Hills soil compared to the Darjeeling soil, which might be explained by the variation in the initial higher amounts of metals as well as the variation in pedogenic processes of soil formation where the dominant pedogenic process was laterization in the case of the Nandi Hills soil, resulting an accumulation of sesquioxides and loss of silica and the reverse is the case with the Darjeeling soil, where the podzolization process is dominant in which accumulation of silica and loss of sesquioxides occurred.26 The amount of DTPA extractable Zn content was deficient (0.51 mg/kg) and marginally deficient (0.61 mg/kg) in the Nandi Hills soil based on the critical level of 0.60 mg/kg. However, such decreased availability of Zn and Cu in soils might be explained by their greater fixation and adsorption as well as greater interaction between soil components.27 The results also reveal that the amounts of DTPA-extractable nonnutrient heavy metals (Cr, Cd, Pb, and Ni) were far below the toxic limit based on the test value.28 The amounts of DTPA-extractable Ni, Cd, Cr, and Pb contents were recorded at very low values in both the soils of Darjeeling and the Nandi Hills, which might be due to the higher organic carbon content in the former soil and higher pH in the latter soil resulting from the complexation of those heavy metals with organic matter in the Darjeeling soil and the higher adsorption of those metals onto sesquioxides in the Nandi Hills soil. The availability of Ni associated with organic colloids is highly pH dependent, which reduced the rate of dissociation of Ni fulvic acid complexes with increased pH and decreased ionic strength.28 The availability of nonnutrient heavy metals such as Cr, Cd, Ni, and Pb and also beneficial micronutrients like Fe, Zn, and Cu in soil might be attributed to the individual soil characteristics, particularly soil pH;29 cation exchange capacity;30 different oxides of Fe, Al, and Mn;31 and amount of organic matter content.32
The results (Table 3) reveal that the amounts of nonnutrient heavy metals (Cr, Cd, Ni, and Pb) in both PD and CO varied between soil types and kind of medicinal plants, being slightly higher in PD compared to CO in both soils. Such low content of those metals in plants might be due to the very low content of those metals in both soils resulting from the variation in soil reaction as well as amount of organic carbon content in the soils.28 Karak et al.33 reported that the concentration of Zn and other nonnutrient heavy metals in soil solution and their availability to crops is controlled by sorption–desorption reactions at the surfaces of soil colloidal materials. The results of the present investigation are similar to those reported by earlier investigators.34,35
However, the overall results reveal that the amount of available trace heavy metals including beneficial and nonnutrient metals depends on the nature and properties of soils, pedogenic processes of soil formation, etc. In the case of the Darjeeling soil, exchangeable Al is mainly responsible for the development of soil acidity, while in the Nandi Hills soil, extensive leaching and at the same time accumulation of sesquioxide are responsible for the acidity. Since all those metal concentrations in their available forms in both soils are very low and the absorption in and uptake of those metals by plants are also reportedly low, based on the results of the present investigation, the cultivation of medicinal plants in both soils is suitable without their medicinal value being affected.
Yields of the extracts
Yields of extracts were determined w/w and tabulated. The yield of extract showed a slightly increased amount procured from the Nandi Hills, Bangalore. This may be due to higher accumulation of Fe, Zn, and Cu and lower content of nonessential heavy metals in leaf samples of CO and PD. An earlier report confirmed this result.19
Antipsoriasis activity
In recent years many plant extracts in combinations or alone have been applied for psoriasis treatments. However, the main concern is to discover new drugs from plant extracts that are more potent than the extracts against any kind of human health hazards. The main reason behind the selection of the leaves of these two plants was that both the plants enhanced the immunity and act strongly against any infections due to their high antioxidant activities.10,36 It was revealed that the selected plant extracts showed significant antipsoriasis activity due to the presence of important secondary metabolites (discussed earlier in the Introduction).
It was scientifically proved that prostaglandin E2 produced by the cyclo-oxygenase pathway results in psoriasis by dilating skin capillaries, which increases leukocyte infiltration and stimulates keratinocyte cell growth.37 During induction of psoriasis, 5% imiquimod cream was used, which showed redness, erythema, and scales within 7 days in the skin of mice. Histologically, psoriatic skin contains a thickened epidermis with a large number of inflammatory cells and absence of a granular layer. In the present study fully developed psoriatic lesions were treated with combined herbal aqueous extracts of PD and CO and compared with marketed standard drug along with untreated animals. The results revealed a significant reduction in epidermal thickness after treatment with the aqueous extracts.
Previous studies have established that antioxidants could play an effective role in psoriasis treatment.38 Plant secondary metabolites such as flavonoids, triterpenoids, and polyphenolic compounds are well known for antioxidant activity and for their anti-inflammatory, antiproliferative, and immunomodulatory activities.39,40 These characteristics of polyphenolic phytoconstituents are beneficial for the treatment of psoriasis and they are present in huge quantities in PD and CO leaves. Preliminary phytochemical screening through chemical tests revealed the presence of these constituents, which showed antipsoriasis activities.
CONCLUSIONS
The results of this present study demonstrate that the combined extracts of PD and CO provide significant antipsoriasis activity and the effect was dose dependent. The selected plants showed remarkable activity compared to that of the marketed standard drug Retino. Hence, further isolation of newer chemicals and clinical trials are needed for the establishment of effective herbal drug formulations against psoriasis via new drug discovery.
ACKNOWLEDGEMENTS
The authors express their sincere gratitude to The Management, Krupanidhi Group of Institutions for funding the project through Krupanidhi Research Incubator Centre (K-RIC) program under Krupanidhi College of Pharmacy and Dr. S. Parthasarathi, Accendere: CL Educate Ltd.
Conflict of Interest: No conflict of interest was declared by the authors.